Discovery
Medicinal Chemistry Services
Discovery ADME
- Metabolic Stability
- CYP Interaction
- Permeability & Transporters
- Physicochemical Properties
- Protein Binding
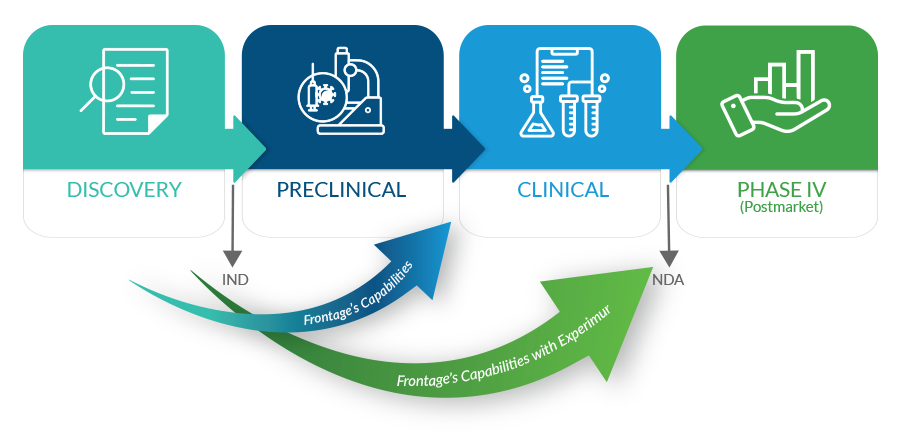
From late discovery through IND preparedness to NDA submissions, Frontage offers a full spectrum of safety and toxicology services. Build confidence in your products early with exploratory toxicology services and fast PK services for lead optimization. Frontage’s integrated safety, bioanalytical, and toxicology services provide complete support and seamless transition to late-stage studies, guiding new therapies from discovery through development to product launch. Our highly skilled and client-focused staff has extensive academic, scientific, and pharmaceutical industry experience. Our studies are conducted in laboratories and suites specifically designed to facilitate a wide range of test species (rodents to primates) and administration routes and are equipped with state-of-the-art instrumentation.

We offer a complete suite of services including Pivotal and Genetic Toxicology, General Toxicology, Toxicokinetic studies, and Safety Pharmacology.
Our extensive profile of IND-enabling pivotal studies supports the swift progression of your lead compounds into the clinic – streamlining your development process.
Schedule a meeting to discuss your upcoming IND-enabling studies.
With Experimur’s acquisition, Frontage now has significantly expanded our range of services, supporting customers’ drug-development programs beyond IND and into NDA-enabling toxicology studies.
Request a quote for specialized toxicology studies.
Experimur/Frontage, Chicago, IL |
Frontage, Concord, OH |
Frontage, Suzhou, China |
|
|---|---|---|---|
Facility |
Custom-designed, purpose-built, 54,000 sq ft | Custom-designed, purpose-built, 85,000 sq ft | >200,000 sq ft |
IND-Enabling Services |
Dose Formulation
|
General Toxicology
Full clinical and anatomic pathology, ophthalmology, ECG |
Dose Range Finding Test
Single-dose & Repeated-dose Toxicity Testing
Local Toxicity Testing Immunogenicity Testing |
NDA-Enabling Services |
Specialized Toxicology
|
– |
|
Animal Rooms |
40 Animal Rooms (23 small, 17 large) | 32 Animal Rooms | 100 Animal Rooms |
In-house Histology and Pathology |
Yes | Yes | Yes |
Accreditations |
|
|
– |
Additional in-house support services include histology, diagnostic pathology, clinical pathology, and analytical chemistry.
Frontage Laboratories, Inc. is committed to the enhancement of animal health and welfare. We honor an organizational commitment toward the ethical and humane use of laboratory animals, paying particular attention to the housing conditions and environmental enrichment of our animal species.
Frontage’s general toxicology group provides all of the resources you’ll need for your investigational plans.
Learn MoreManaging an Investigational New Drug (IND) program and submission is a daunting task, requiring extensive planning and ongoing coordination of multiple scientific disciplines, each interrelated and reliant upon the other to achieve each successive milestone.
Learn MoreIn order to initiate clinical trials for women of child-bearing potential (WOCBP), the FDA requires initiation of preclinical developmental and reproductive toxicity (DART) studies using mammalian research models.
Learn More